NewroBus™
Carrying Biologics Across the Blood-Brain Barrier
A Novel Approach to Alzheimer’s Disease
Our Mission
Developing novel treatments for Alzheimer’s Disease and other neurodegenerative diseases by carrying powerful biologics across the Blood Brain Barrier (BBB)
Our Technology
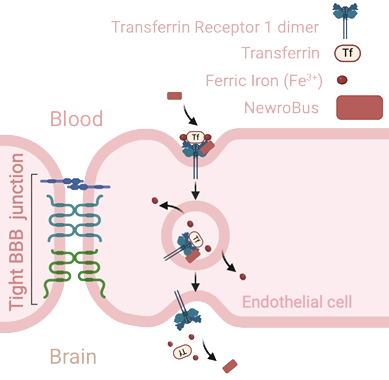
Versatile and patented platform based on single-chain nanobodies exploiting Transferrin Receptor 1 (TfR1) mediated transcytosis to carry biologics efficiently and safely across the BBB.
NewroBus™
Exploiting Transferrin Receptor 1 (TfR1) transcytosis to carry biologics through the BBB
- High humanness and specificity
- High BBB permeability (CSF/Serum ratio >0.90)
- High tolerability

Alzheimer Disease and TNF-α
Tumor necrosis factor α (TNF-α) plays a central role in the pathophysiology of Alzheimer’s Disease (AD). FDA–approved biologic TNF-α inhibitors are potential treatments for AD, but they do not cross the blood-brain barrier.
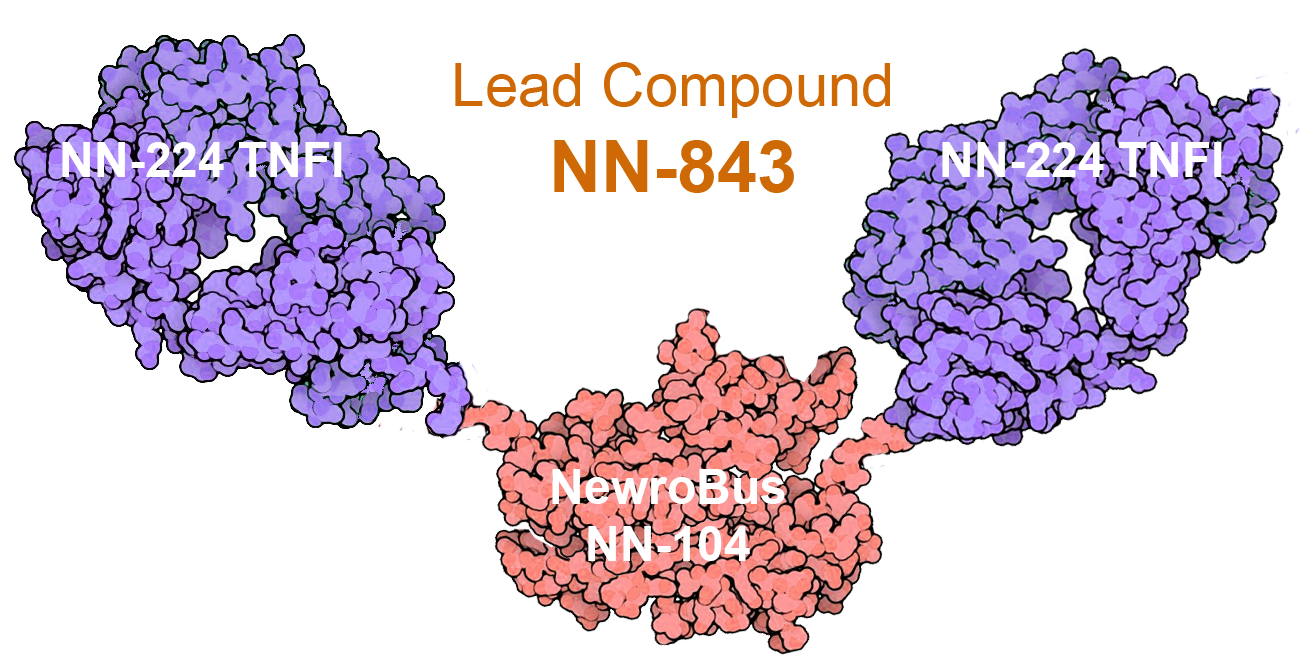
Our Lead Product: NN-843
Heterotrimer tested in animals consisting of NewroBus NN-104 linked to two molecules of our proprietary TNF-α inhibitor NN-224
- NN-843 crosses the BBB after subcutaneous administration
- High CSF/Serum ratio and broad brain distribution
- Potent TNF-α inhibition
- Excellent tolerability